Fire Research
Supporting Science & Technology
A microscale combustion calorimeter has been developed to measure flammability parameters of milligram polymer (plastic) samples under conditions which approximate aircraft cabin fires. The test takes only a few minutes to run and provides a quantitative measure of the aircraft fire hazard of research materials which are only available in milligram quantities from university and industrial polymer scientists. Quick feedback on flammability of new materials has helped guide the synthetic effort towards improved fire resistance. Figure 1 is a photograph of the microscale combustion calorimeter. A sharp, quantitative, and reproducible heat release rate peak is obtained in the microscale heat release rate test. After normalizing the curves for the sample size the results are independent of the physical form of the material (e.g., powder, film, fiber, etc.). The microscale heat release rate data are expressed in kilowatts per gram of original material.
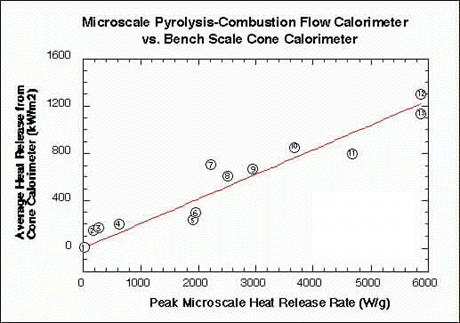
Figure 2 compares the peak microscale heat release rate (HRR) measured on milligram samples of pure plastics in the microcalorimeter to the heat release rate measured for 100 gram samples of the same material in a conventional fire calorimeter . The heat release rate plotted along the vertical axis is the steady-state or average value obtained in a fire (cone) calorimeter at 50 kW/m2 incident heat flux according to standard procedures.
FIGURE 1. FAA MICROSCALE PYROLYSIS-COMBUSTION FLOW CALORIMETER (A) AND BENCH SCALE CONE CALORIMETER (B).
FIGURE 2. CORRELATION BETWEEN MICROSCALE CALORIMETER AND BENCH SCALE CONE CALORIMETER RESULTS FOR HEAT RELEASE RATE OF PLASTIC MATERIALS USED IN AIRCRAFT.
Full-scale fire tests at the FAA have shown that the heat release rate of interior materials measured in a fire calorimeter correlates with passenger escape time in a simulated post crash fuel fire. The good correlation between fire- and micro-calorimeter results shows that the microcalorimeter is also a good predictor of passenger escape time and, therefor, of full-scale fire hazard. A DOT/FAA patent has been filed on this invention (FAA/Galaxy Scientific Corporation).
Figure 3 shows microcalorimeter results for some of the new materials being devoloped in the program and how they compare materials currently being used in aircraft. It is clear that most of the new materials tested show significantly lower heat release rate when combusted than do the current (baseline) aircraft materials.
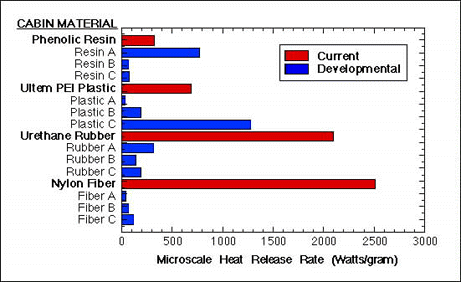
FIGURE 3. HEAT RELEASE RATE OF CURRENT AND DEVELOPMENTAL AIRCRAFT CABIN MATERIALS.
In addition to creating a metric for materials development (i.e., the microscale calorimeter) Supporting Science and Technology provided the following technology base:
- Thermal degradation of polymers was modeled by computer to study the process which generates combustible fuel in a fire. The computer code (MD REACT) uses a commercial program (Discover 95, Molecular Simulations) to create the polymer chains for the model and then simulates the thermal degradation process at fire temperatures. This code will provide insight into the process and products of thermal degradation to allow the design of thermally-stable, less toxic, fire-resistant polymers (NIST)
- A mechanistic fuel generation model was developed for polymers in fires which includes the competing processes of gasification and char formation. The simple analytic result provides the fuel generation rate of a burning polymer from conventional laboratory thermal analysis data (FAA W. J. Hughes Technical Center).
- Molecular modeling of flammability utilizes computational quantum chemistry to predict the thermochemistry and kinetics of solid- and gas-phase combustion processes of burning polymers. Initial results for the effects of chain size and stereoregularity on the bond dissociation energy of novel polycarbodiimides were obtained (U. of Massachusetts, Amherst).
- Atomistic models of polybenzoxazine have been developed and solved on a computer which provide insight into the high thermal stability and unusual volumetric expansion on polymerization (U. of Akron).
- Computational modeling of intumescence has shown the importance of bubble nucleation kinetics and thermal properties on the insulation value of this important commercial fireproofing technology (NIST)
- Thermomechanical stability of fire exposed resins and composites are studied using a new noncontact technique-ultrasonic spectroscopy. Results indicate surface embrittlement of the resin in a fire initiates surface cracks which mechanically destabilize the composite (U. of Massachusetts, Amherst).
- An integral method of nonisothermal kinetic analysis provides a new, semi-exact solution of the mass loss integral which is useful for extracting flammability parameters from laboratory thermal analysis data (FAA W. J. Hughes Technical Center).
- In-situ flame chemistry by remote laser spectroscopy has been successful at identifying flame species in burning polymers to study the chemical reactions which lead to combustion inhibition and toxic by products in flame retarded polymers. A patent is being filed on this technology (U. South Carolina)
- Fire calorimetry of flame retardant polymers showed that fire retarded (FR) polymers ignited and burned readily under aircraft cabin fire exposure conditions with increased smoke and toxic gas production compared to the original (non-FR) material- validating the FAA¹s focus on inherently fire resistant polymers and composites (Omega Point Labs).
 Federal Aviation Administration
Federal Aviation Administration